Ab initio molecular dynamics;Properties of Water and Hydrophobic EffectThe polar nature of water, with its partial positive and partial negative dipole, explains why bulk water readily dissolves many ionic species and interacts with ionic surfaces The difference between isolated vapor phase water and bulk liquid water is much more extreme than can be accounted for by a model relying only on dipole interaction

Hydrophilic Directional Slippery Rough Surfaces For Water Harvesting Science Advances
Hydrophobic nature of water
Hydrophobic nature of water- Our understanding of the hydrophobic effect has advanced greatly since 1990, with the help of experimental, theoretical, and computer simulation results The key hydrophobic signature of positive ∆C°P and negative ∆S° at room temperature has been interpreted in light of the importance of solvent cavity creation, solventexcluded volume, and solute–water Students are introduced to superhydrophobic surfaces and the "lotus effect" Water spilled on a superhydrophobic surface does not wet the surface, but simply rolls off Additionally, as water moves across the superhydrophobic surface, it picks up and carries away any foreign material, such as dust or dirt Students learn how plants create and use superhydrophobic surfaces in nature




The Transient Nature Of Water Repellency Caused By Download Scientific Diagram
Waterrepellency in nonwetting sands is due to hydrophobic waxes present on the surface of sand grains and contained in particulate organic matter present in these sands This study investigates the physicochemical characteristics of these natural waxes and compares them to waxes extracted from potential original source materials Hydrophobic nature of the majority of polymers though suggests that the presence of hydrophilic water should not impact selfhealing properties ForMolecules forming ionic or a hydrogen bond with the water molecule are said to be hydrophilic This property of water was important for the evolution of life
Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned close to one another Such associations are vital for the structure of the components of microorganisms Hydrophobic ("water hating") interactions are created because of the uncharged nature of the involved chemical groups Study and reproduce the superhydrophobic and superoleophobic characters will benefit various potential applications All we know that lotus leaf is the best example for superhydrophobic and selfcleaning demonstration in nature Studies found that many of the plants with waterrepellent properties have threedimensional wax crystals at nanoIn this video I show you how I made hydrophobic water!
Water striders are insects that live on the surface film of water, and their bodies are effectively unwettable due to specialized hairpiles called hydrofuge;The interaction of water with small alcohols can be used as a model for understanding hydrophobic solvation of larger and more complex amphiphilic molecules Despite its apparent simplicity, water/ethanol mixtures show important anomalies in several of their properties, like specific heat or partial molar vo PCCP HOT ArticlesAs the mimic biology becomes more and more important in the field of technology, superhydrophobic materials in the natural world have also become common Superhydrophobic surfaces are used to prevent water droplets from wetting themselves which contain the micro and nanostructures named hierarchical surfaces and exhibit the high water contact angles (WCA)




Modeling Super Hydrophobic Surfaces




What Makes A Surface Waterproof Resin Library
HYGs, because of their hydrophilic nature, have very low contact angles for water NCHYGs, however, can have particular polymer–nanoparticle links that enhance hydrophobicity In fact, Haraguchi et al have found that the surface of an NCHYG consisting of a PNIPAAm/clay network exhibits extraordinarily high hydrophobicity and showed a maximum contact angle of 151 Main Difference – Hydrophobic vs Hydrophilic Molecules Water is a wellknown solvent for the dissolution of most of the compounds we know But all compounds in nature do not mix with water The substances that can mix with water are called hydrophilic substances; Wrapping up hydrophobic hydration Solvent chemistry Date Source RuhrUniversity Bochum Summary Studied in detail, the embedding of hydrophobic molecules in water looks quite




Quantum Destabilization Of A Water Sandwich Kaust Discovery




Fluidity And Phase Transitions Of Water In Hydrophobic And Hydrophilic Nanotubes Scientific Reports
A nonionic hydrophobic natural deep eutectic solvent (HNADES) based on thymol and menthol was proposed for the liquidliquid microextraction of fourteen phthalates and one adipate from environmental water samples Separation, identification, and quantification were achieved by ultrahighperformance liquid chromatography coupled to tandem mass spectrometryHydrophobic molecules Water, H 2 O, is a polar molecule, that is, it has polarity, which is an uneven distribution of electron density among its atomsThe oxygen side of any water molecule is slightly negative, while the hydrogen side is slightly positive Polar water does not bond with nonpolar or hydrophobic molecules Insert picture such as this one retrieved from https//wwwNonpolar molecules that repel the water molecules are said to be hydrophobic;



Water Repellency




Hydrophobic Stock Video Footage 4k And Hd Video Clips Shutterstock
Despite its strongly hydrophobic character, the initially empty central channel of the nanotube is rapidly filled by water from the surrounding reservoir, and remains occupied by a, Deflection of water jets from the superhydrophobic surface before (left) and after (right) abrasionThis is a form of waterproof water that can actually repel normal waterFollow me on Twitter https//




Hydrophobic Organosilicacoatingonsteelandaluminum




Hydrophilic Directional Slippery Rough Surfaces For Water Harvesting Science Advances
Initially, at P = 0 MPa (I stage in Fig 1C), no water molecules are intruded into nanopore, indicating the hydrophobic nature of the C8modified nanopore wall surface Upon moving the piston, when P increases to a critical pressure P i n (∼145 MPa, determined by the midpoint of the plateau of the curve, II stage in Fig 1 C ), liquid molecules overcome the capillary The hydrophilic adjective describes the character of a molecule or atomic group that has an affinity for water and the hydrophobic adjective describes the character of a molecule or atomic group that is insoluble in water, or resistant to wetting or hydrationDespite its great importance in numerous phenomena, the origin of hydrophobicity remains one of the most disputed topics in science (1 ⇓ ⇓ ⇓ –5)Experimental studies have shown that small purely hydrophobic solutes (alkanes and noble gases) in water
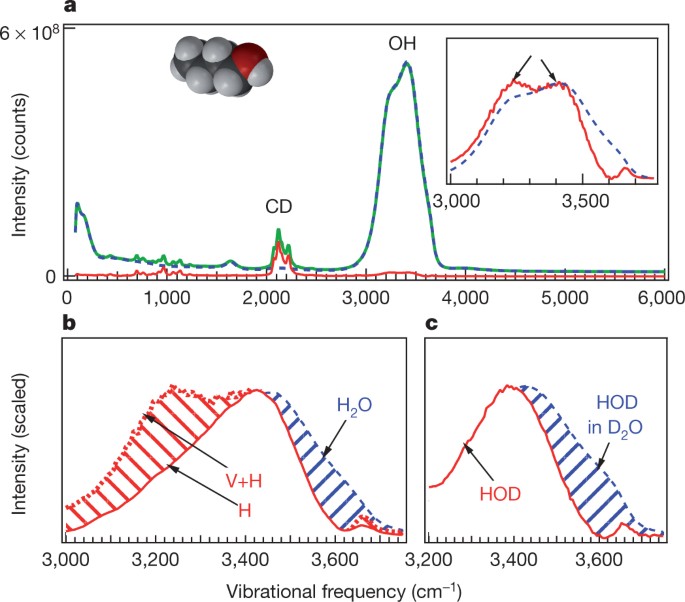



Water Structural Transformation At Molecular Hydrophobic Interfaces Nature
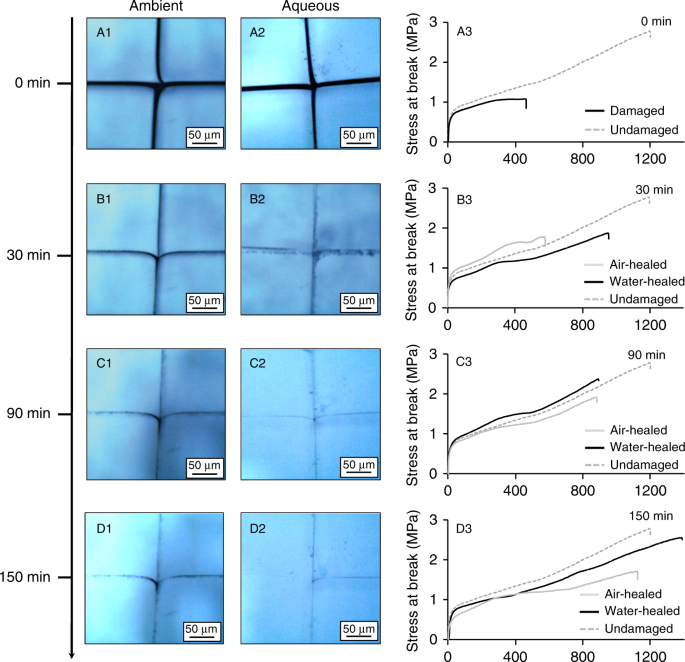



Water Accelerated Self Healing Of Hydrophobic Copolymers Nature Communications
Water is used as an indicator to measure how hydrophobic and/or hydrophilic a coated surface is As such, it's important to understand the nature of water Water is a polar molecule, which means it carries a partial charge between atoms In a water molecule (H 2 O), the oxygen molecule is more#BYU mechanical engineering professors Julie Crockett and Dan Maynes study superhydrophobic surfaces, or surfaces that are extremely difficult to get wet In This leaves the hydrophobic tails touching the inside of the circle However, the intracellular environment of a cell is aqueous, or waterbased So the tails aren't going to like this




Beading Effect Illustrating The Hydrophobic Water Repellent Nature Download Scientific Diagram




What Are Hydrophilic And Hydrophobic Molecules Quora
The substances that cannot mix with water are known hydrophobic substancesMany of their body surfaces are covered with these specialized "hairpiles", composed of tiny hairs spaced so closely that there are more than one thousand microhairs per mm, which creates a hydrophobic surface Physicists discover a paradox hydrophobic water Now you can extend that truism about oil and water to water and itself Water and water don't always mix, either The textbooks say that water




Unravelling The Mysteries Of Carbonic Acid Berkeley Lab



Hydrophobic Coatings Super Hydrophobic Nano Coating Treatments
The superhydrophobic nature of surfaces is greatly dependent on the interfacial molecular structure of coating materials In this study, to understand the structure, dynamics and interfacial behavior of hydrophobic coating, molecular dynamics is utilized to study the capillary transport of water molecules t 19 PCCP HOT Articles Hydrophobicity Two faces of water Water and oil famously don't mix the term hydrophobic (waterfearing) is commonly used to describe substances that, like oil, do not mix with water AlthoughWater superhydrophobic Hydrophobic Surfaces "Waterfearing surface" Water tries to minimize contact with surface Examples Teflon, oily surfaces water hydrophobic surface Hydrophilic Surfaces "Waterloving surface" Water tries to maximize contact with surface Examples Glass, rusted metal surfaces water hydrophilic surface




Hydrophobic Effect An Overview Sciencedirect Topics




Hydrophilic And Hydrophobic Surface Stock Vector Illustration Of Education Hydrophobic
Hydrophobic amino acids are a type of amino acids with a nonpolar nature Likewise, the name "hydrophobic" derives because it does not interact with water ("hydro" – water) Water is a polar solvent Since these amino acids are nonpolar, they cannot dissolve in waterWater has a very high specific heat capacity of 4184 J/(kg·K) at 25 °C – the secondhighest among all the heteroatomic species (after ammonia), as well as a high heat of vaporization (4065 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of the extensive hydrogen bonding between its molecules These two unusual properties allow water to moderate Earth'sFrom Wikipedia, the free encyclopedia A superhydrophobic coating is a thin surface layer that repels water It is made from superhydrophobic (ultrahydrophobicity) materials Droplets hitting this kind of coating can fully rebound




The Lotus Leaf How Nature Makes Water Repellent Materials



What Is Water And What Are Hydrophobic And Hydrophilic Surfaces Steemit
A readytouse water dispersible pigment composition containing at least 5% by weight of water is provided The composition comprises a stable dispersion of a waterinsoluble and/or hydrophobic natural pigment such as a carotenoid, curcumin, a porphyrin pigment or vegetables carbon black in the form of bodies of an average size which is at the most 10 μm is provided This waterinduced attraction between oil molecules is called the hydrophobic interaction In the 1950s, Walter Kauzmann identified hydrophobic interactions as a primary source of protein stability The most famous example of natural superhydrophobic surfaces are lotus leaves (Nelumbo nucifera), which are characterized by θw > 150°, ultralow water adhesion (ultralow H and α) and selfcleaning properties (Fig 2)




3d Imaging Of Water Drop Condensation On Hydrophobic And Hydrophilic Lubricant Impregnated Surfaces Topic Of Research Paper In Nano Technology Download Scholarly Article Pdf And Read For Free On Cyberleninka Open Science Hub




What Are Hydrophilic And Hydrophobic Molecules Quora
A nonionic hydrophobic natural deep eutectic solvent (HNADES) based on thymol and menthol was proposed for the liquidliquid microextraction of fourteen phthalates and one adipate from environmental water samples Separation, identification, and quantification were achieved by ultrahighperformanc144 Hydrophobicity of Adsorbent Material When hydrophobic particles are suspended in water, they have tendency to interact with particles of their kinds rather than with water Typical example is the oil droplet, because of this trend, hydrophobic particles suspended in water has the physical nature of voids in the bulk solutionWhile nature can master the superhydrophobic surface with relative ease, it is far more difficult to create superoleophobic surfaces that can also resist oils and superomniphobic surfaces




Topic 2 Molecular Biology 2 2 Water Ib




3p1 1 Chapter 3 Outline 1 Molecular Nature Of Water Noncovalent Bonding Ionic Interactions Hydrogen Bonds Van Der Waals Forces Thermal Properties Of Water Ppt Download
A hydrophobic material is one that repels water Inspiration for these materials tends to come from nature – for a long time the best hydrophobic materials worked on the same principle that allow lotus leaves to float on water without becoming waterlogged (the leaves have a rough surface that minimises the contact area between the liquid and solid)Natural Organic Matters (NOM), which are abundant in water resources, are considered as strong foulants during water treatment with membrane processes (Zazouli et al, 08a, and 08b) The NOM can be broadly divided into two fractions of hydrophobic fraction such as humic acid and hydrophilic fraction such as alginic acid (Zazouli et al, 07) Due to the hydrophobic nature of the coating, water remains upon the roughness and contacts a mixture of solid and air, as evidenced by the silvery aspect of its base Using the Cassie formula, we can deduce from contact angles the proportion ϕ of solid/water contact and find ϕ ∼ 35 ± %, a value much smaller than unity
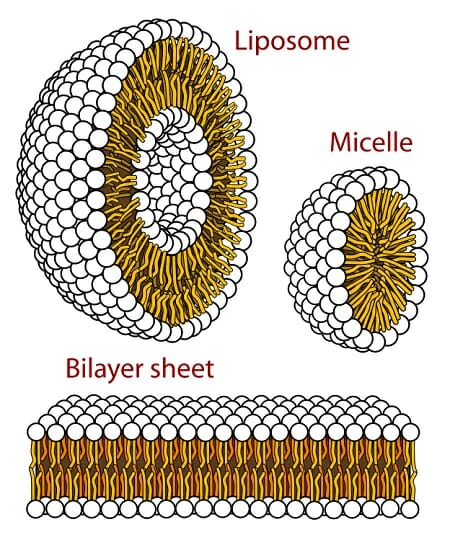



Hydrophobic Definition And Examples Biology Dictionary




Water Shedding Surfaces Can Be Made To Last
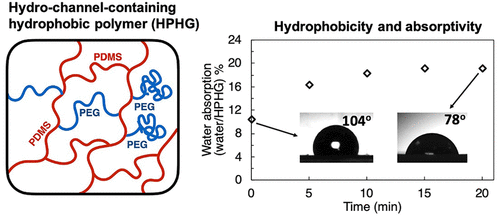



Hydrochannel Containing Hydrophobic Polymers By Inverse Emulsion Polymerization For Moisture Driven Actuators Acs Applied Materials Interfaces X Mol
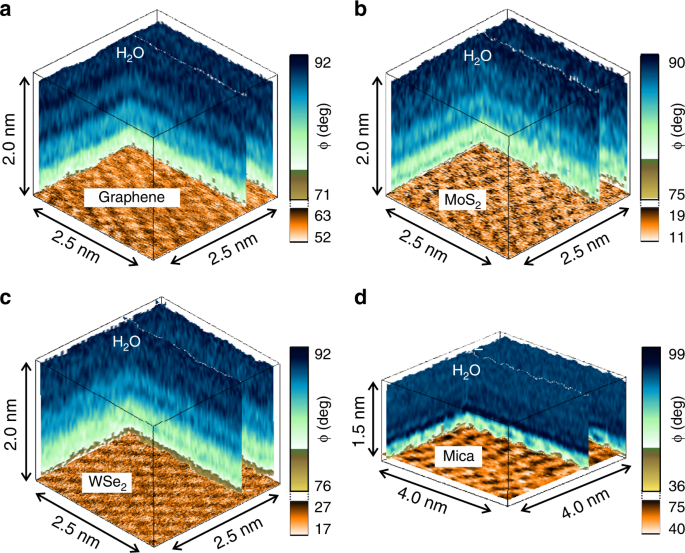



Atomic Scale Mapping Of Hydrophobic Layers On Graphene And Few Layer Mos 2 And Wse 2 In Water Nature Communications




Hydrophobic Organosilicacoatingonsteelandaluminum




Solved Phospholipids Molecules Have A Two Face Nature T Chegg Com
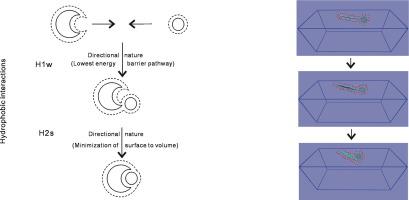



Directional Nature Of Hydrophobic Interactions Implications For The Mechanism Of Molecular Recognition Chemical Physics X Mol



Is Polar Surface Always Hydrophilic Shanghai Institute Of Applied Physics Chines Academy Of Sciences




Hello Materials Blog Nature Inspiration Biomimicry Architecture Hydrophobic



1




1 600 Hydrophobic Photos Free Royalty Free Stock Photos From Dreamstime




Lipids The Lipids Are Organic Molecules Present In Nature Are Insoluble In Water Hydrophobic While They Are Soluble In Organic Solvents Non Polar Ppt Download




The Transient Nature Of Water Repellency Caused By Download Scientific Diagram




Insects Inspire Water Repellent Material Physics World




O Oo Environment A Royalty Free Stock Photo From Photocase




Beading Effect Illustrating The Hydrophobic Water Repellent Nature Download Scientific Diagram



2




Hydrophobic Definition And Examples Biology Online Dictionary




Hydrophobe Wikidoc



1




Kupit Rain X Treme Glass Care Water Repellant Hydrophobic Na Aukcion De Iz Germanii S Dostavkoj V Rossiyu Ukrainu Kazahstan




The Hydrophobic Nature Of Brussels Sprouts Combined With Water S High Surface Tension Makes The Water Look Like Jelly Gardening




Ride Of The Water Droplets Watch How Weirdly Water Behaves On Hydrophobic Surfaces Adafruit Industries Makers Hackers Artists Designers And Engineers




Hydrophobicity Will The Drop Stop Or Roll Science Friday




A Sketch Of Water Drops On A Hydrophilic A Hydrophobic And A Download Scientific Diagram




Hydrophobic Stock Video Footage Royalty Free Hydrophobic Videos Pond5




Hydrophobic Effect An Overview Sciencedirect Topics




Solved Which Of The Following Characteristics Describes W Chegg Com
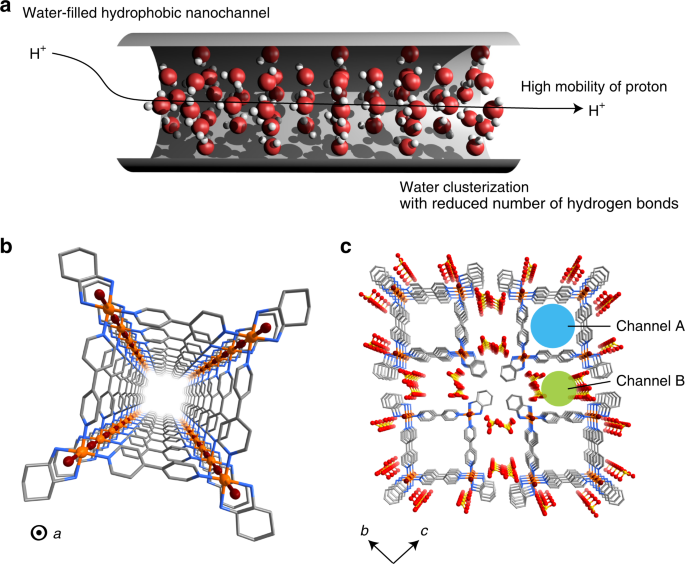



Confined Water Mediated High Proton Conduction In Hydrophobic Channel Of A Synthetic Nanotube Nature Communications



1




Phase Transfer Of Hydrophobic Qds For Water Soluble And Biocompatible Nature Through Silanization Sciencedirect



Difference Between Hydrophobic And Hydrophilic Molecules Definition Properties Examples




Configurations Of Liquid Water Molecules Near Hydrophobic Cavities In Download Scientific Diagram




Plants Nature Natural Science Hydrophobic Action Of Water Droplets On A Plant Leaf Beading Stock Photo Alamy




Water The Medium Of Life Ppt Download
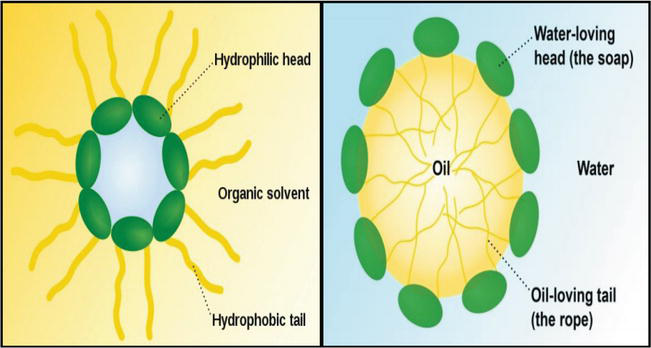



Hydrophobic Polymers Flooding Intechopen



1




Hydrophobic Effect Wikipedia




First Drop Water Droplet Hydrophobic Nature Stock Photo Edit Now




Hydrophobic Organosilicacoatingonsteelandaluminum



Hydrophobicity Two Faces Of Water Nature




Hydrophobic Stock Video Footage Royalty Free Hydrophobic Videos Pond5




Schematics Showing The Hydrophobic Nature Of A Fluorinated Flat Sio 2 Download Scientific Diagram
:max_bytes(150000):strip_icc()/olive-oil-bubbles-in-water--close-up-sb10062488l-001-596932675f9b582c356d5411-5c5209594cedfd0001f912a0.jpg)



The Definition Of Hydrophobic With Examples



Hydrophobic Interactions Among Dissolved Molecules Course Hero




The Water Race Hydrophobic Hydrophilic Surfaces National Nanotechnology Infrastructure Network




Intermolecular Forces Van Der Waals Force Hydrogen Bond Hydrophobic Effect Ryosuke University




Incredibly Hydrophobic Surfaces Teaching Biology Homeschool Science Science And Nature




Hydrophilic Definition And Examples Biology Online Dictionary




Explained Hydrophobic And Hydrophilic Mit News Massachusetts Institute Of Technology




Ultrafiltration And Nanofiltration Membrane Fouling By Natural Organic Matter Mechanisms And Mitigation By Pre Ozonation And Ph Sciencedirect




Hydrophobic Effect Wikipedia




Explained Hydrophobic And Hydrophilic Mit News Massachusetts Institute Of Technology



What Is Hydrophobic Definition Interactions Video Lesson Transcript Study Com




Influence Of The Hydrophilic Hydrophobic Nature Of Polyetheramines On The Interaction Between Amine Alcohol Silicate Hybrids And Anionic Dyes For Effective Water Cleaning Journal Of Materials Chemistry A Rsc Publishing



Superhydrophobic Materials From Nature Feature Chemistry World




Hydrophobic And Superhydrophobic Coatings Explained




Photos Illustrating The Hydrophobic Nature Of The Skin Of Sea Kraits Download Scientific Diagram




Recent Progress In Understanding Hydrophobic Interactions Pnas




What Is Hydrophobic Definition Interactions Video Lesson Transcript Study Com




Learn About Hydrophobic Substances Chegg Com



Hydrophobic Nano Paint Superhydrophobic Coating
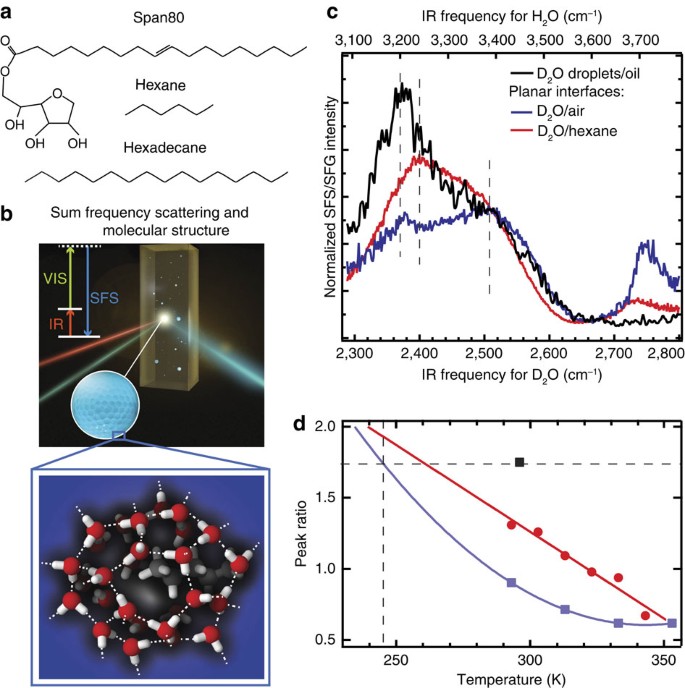



The Interfacial Structure Of Water Droplets In A Hydrophobic Liquid Nature Communications




Hydrophobic Stock Video Footage 4k And Hd Video Clips Shutterstock




Untitled Beautiful Calm A Royalty Free Stock Photo From Photocase




Hk9egxbfkvth6m




Recent Progress In Understanding Hydrophobic Interactions Pnas




Series Of Images Showing The Hydrophobic Nature Of Rac Images Of Water Download Scientific Diagram




57 Hydrophobic Ideas Hydrophobic Macro Photography Flowers Hydrophobic Effect




Contact Angle Wikipedia




Plants Nature Natural Science Hydrophobic Action Of Water Droplets On A Plant Leaf Beading Stock Photo Alamy




74 Hydrophobic Stock Videos Royalty Free Hydrophobic Footage Depositphotos




Superhydrophobic Materials From Nature Feature Chemistry World




What Is Hydrophobic Definition Interactions Video Lesson Transcript Study Com




Hydrophobe Wikipedia




Hydrophobic Or Hydrophilic Aero Gallium Nitride Is Both Physics World




Hydrophilic Hydrophobic Assignment




Hydrophobic Definition And Examples Biology Online Dictionary




Hydrophobic Expanded Perlite




Ak Lectures Properties Of Water And Hydrophobic Effect



Hydrophobic Nano Paint Superhydrophobic Coating
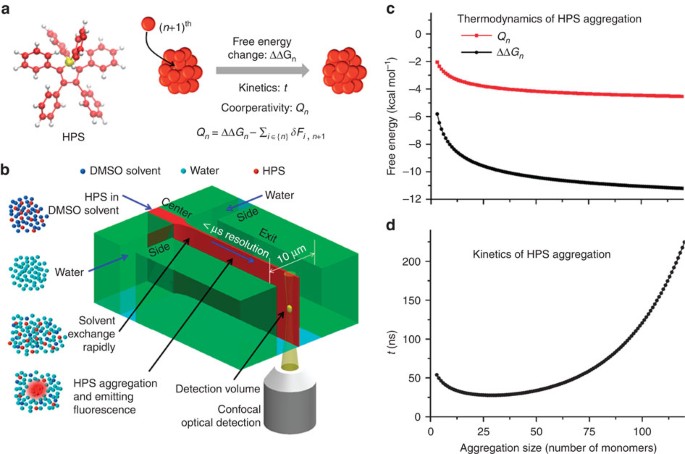



Real Time Monitoring Of Hydrophobic Aggregation Reveals A Critical Role Of Cooperativity In Hydrophobic Effect Nature Communications



0 件のコメント:
コメントを投稿